Are these really potassium iodide and salt crystals?
I only just noticed the suspect features in these SSKI crystals mixed with table salt while going through old sample pictures.
These look very similar to Dr nixon and Dr ana Milhhalcea’s anestheic ventures of late. Sterile water was used and i often check that water source in the usual way one would a control sample. I realize now i shall have to go and look at the salt and iodide seperately to see which one contains the contaminents. I suspect the table salt obviously. The Potassium iodide was from an industrial manufacturer in crystal form to ensure it was clean, But now i am not so sure which is the culprit.
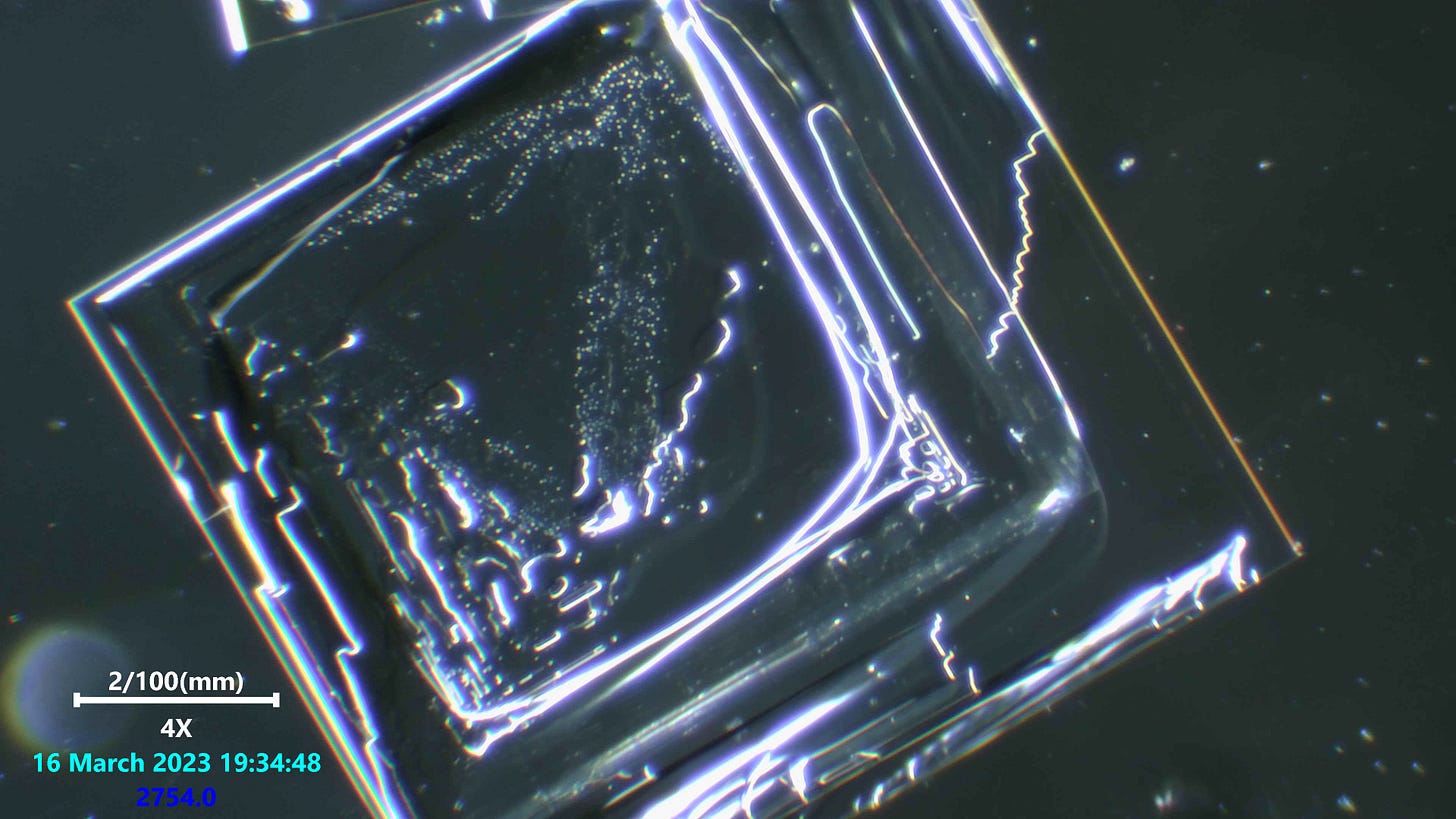
Note the very fine ridge details forming step like features. Absolutely stunning precision unfolding with such tiny detail.
This was the weirdest potassium iodide and salt viewing i have done. I have looked at 2 or 3 brands before this and never saw anything like this. The details are so intricate and really i was surprised when i saw our dot friends present too.
Really not much more to say on this since we have seen these kinds of formations alot and still have no certainty on what they are in any real detail, or what they do. But indeed most of us suspect these structures to be some kind of nano technology based attempt at mimicing electronic devices once they are fully formed and have fibers connected to them. Maybe they do not need a fiber connected to them, but we have seen that happening several times and Dr Nixon recently did a viewing on an indian anesthetic which showed one of these freaky fiber optic like fibers attached to a crystal structure with the Q-dot looking structures and more all lit up.

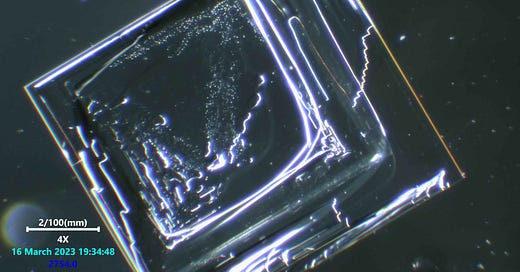


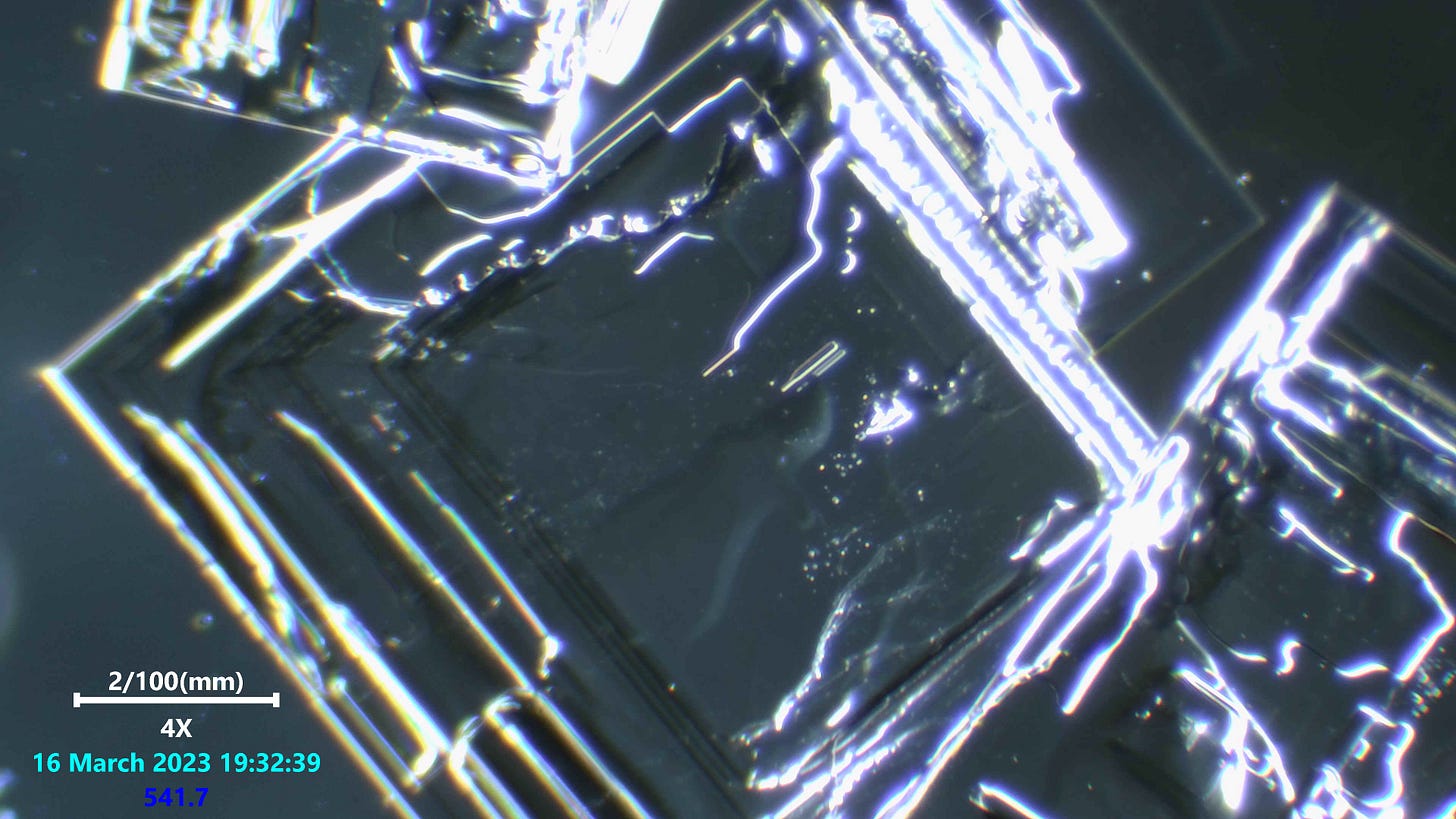
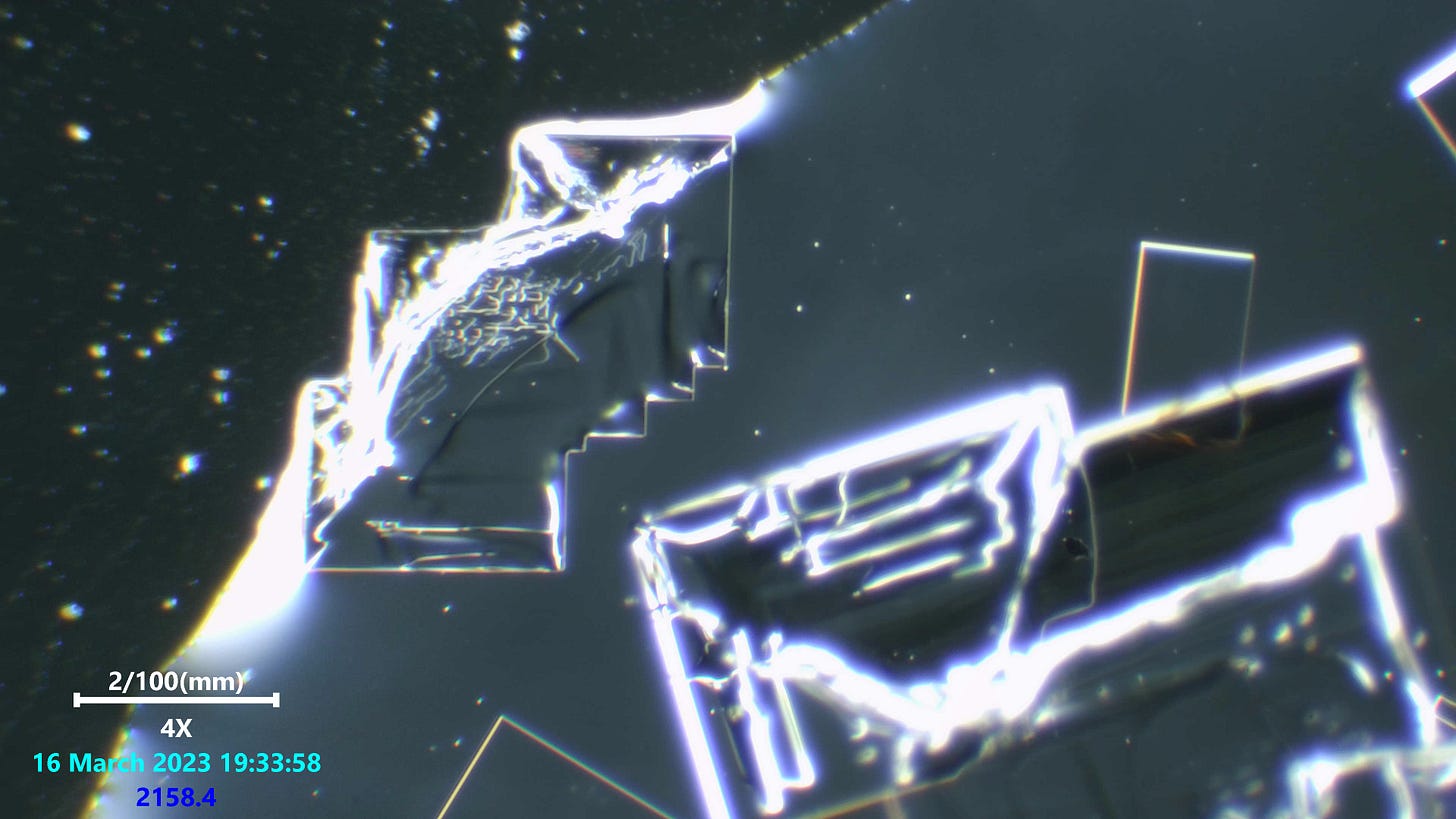
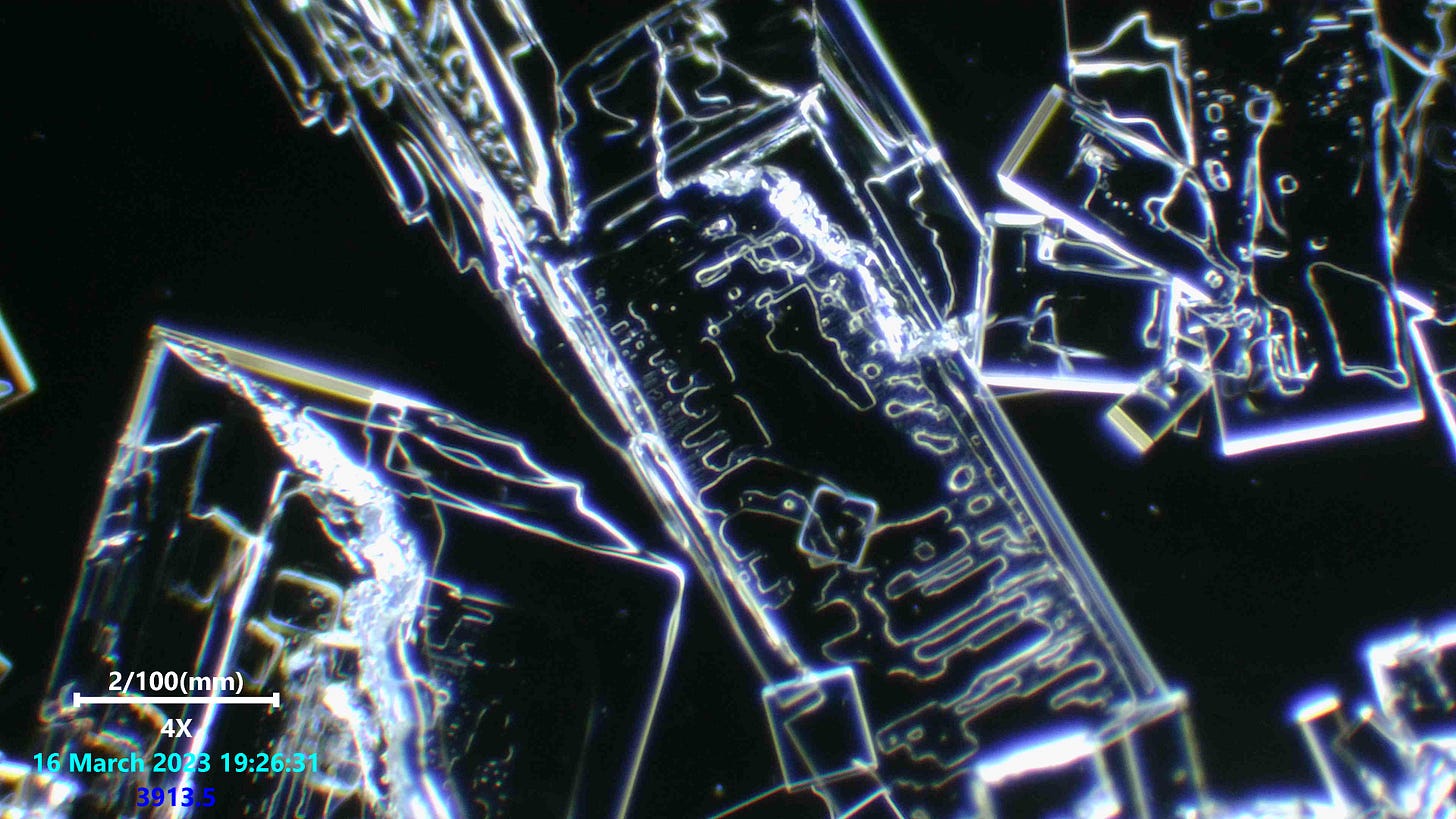
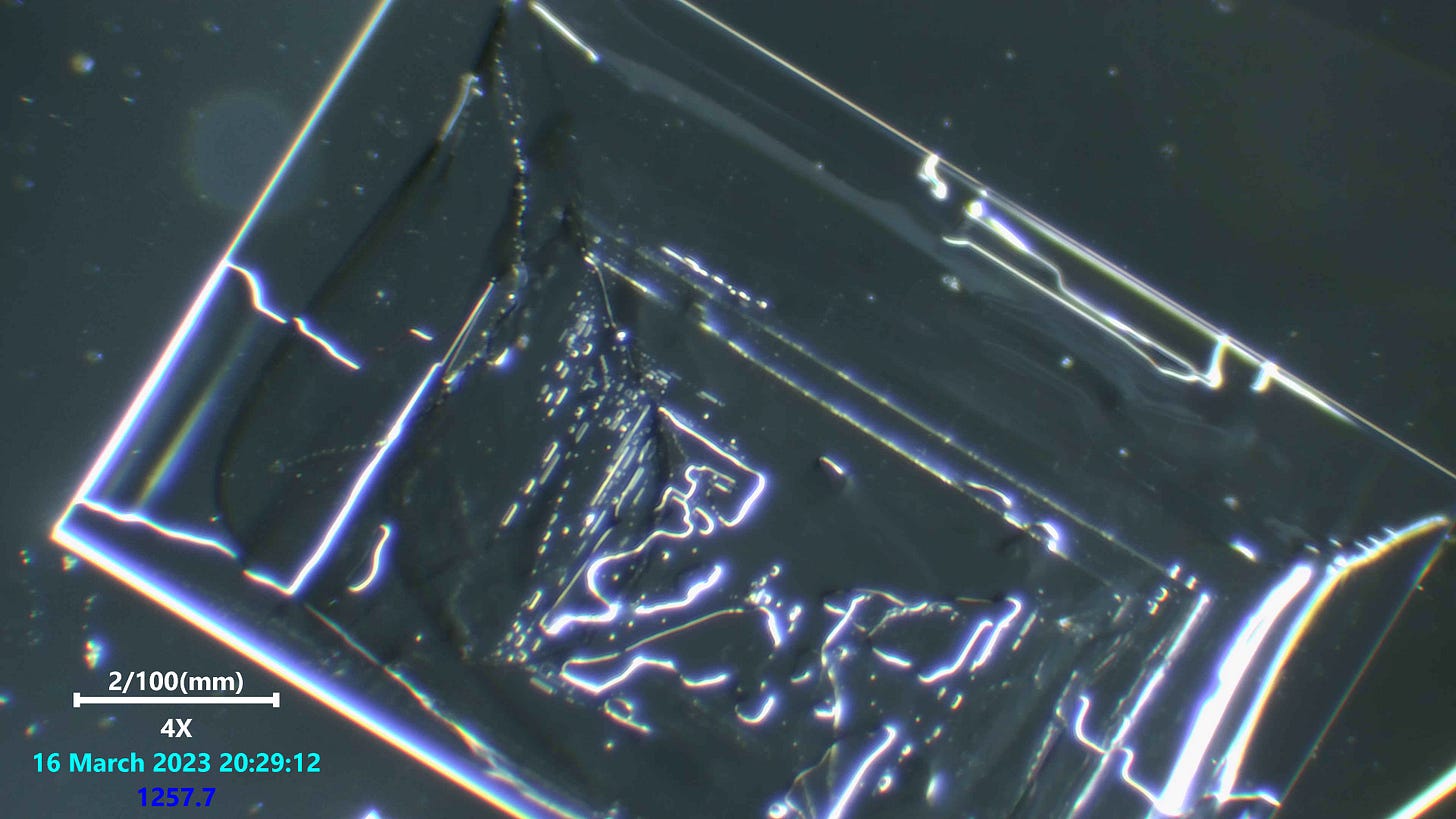
salt crystals are perfectly straight macroscopically because single salt molecules are cubic. Additional molecules can only bond to one the six faces of the cube. So, no matter how big the crystal becomes, the edges will always be straight or at right angles to each other.
I make my iodine sski lugols and elemental iodide
I know China supplies much of the iodine prills that aré imported and used to mass manf iodine
I’ll put some on a slide 🛝 soon
Sorry but can someone explain exactly what was being looked at? Table salt? And manufactured salt crystals? Okay, I’ll read it all again. This is crazy to see this in salt. 🙈
How does this happen?